Rocks
By Roger Russell
Here are some of
the rocks and fossils I collected over the years.
Some I personally picked up in my travels and others I purchased.
All were of interest to me for one reason or another.
![]()

When lightning strikes the ground, another natural phenomenon occurs It forms a glass like artifact that is sometimes hollow and is called a Fulgurite. When lightning strikes sand, it is heated to the point where the sand melts and fuses along the path or paths of the current. The top two sand-based Fulgurites in the picture are about 3 inches long. The bottom one is about3-1/2” long and is from soil found in Morocco. Central Florida is almost all sand. I can dig down as far as I can in my back yard, or front yard, and all I can find is nice clean white sand.
Fulgurites are very fragile, but if dug up carefully, can be found to be arranged like the roots of a tree and be many yards long. Fulgurites can be found almost anywhere in the world where lightning strikes the ground, whether it is sand, dirt or rock. The trick is in finding them, recognizing what they are, and excavating them without too much breakage. These samples weigh about 5 grams each.
![]()
 The first atomic bomb detonation was
on July 16, 1945 in Trinity, New Mexico. The intense heat formed a
grayish-green, glass-like material from the fireball and fused the desert sand
into a jade green crater 2400 feet in diameter. Nearly 2000 tons of intensely
radioactive material was formed. It was mostly silica with traces of olivine
and feldspar.
The first atomic bomb detonation was
on July 16, 1945 in Trinity, New Mexico. The intense heat formed a
grayish-green, glass-like material from the fireball and fused the desert sand
into a jade green crater 2400 feet in diameter. Nearly 2000 tons of intensely
radioactive material was formed. It was mostly silica with traces of olivine
and feldspar.
As soon as soil radiation dropped to safe levels, Trinitite pieces began to disappear into the hands of collectors and visitors looking for mementos. In 1975, Trinity was declared a national historic site and removing Trinitite was strictly prohibited. This sample of Trinitite is still radioactive but is only in the range of 0.3 to 0.5 mR/hr and is harmless. It is 7/8” wide and weighs 3.3 grams.
![]()

The mother of all glass-sand fusion is found near the Libyan-Egyptian border. This action occurred in the North African desert about 28.5 Million years ago. The glass is translucent yellow. The translucence may be caused by a white silica mineral found in volcanic rock and created at very high temperatures. One side shows thumbprint-like impressions similar to portions of the surface of a meteor that are vaporized as it passes through the earth's atmosphere. These in turn could be formed by vortices of hot gas. However, the underside is rough having not been exposed to wear, indicating it may have been molten when it landed on the ground some distance from impact at ground zero. Then, the thumb impressions could have been caused by wind erosion over a very long period of time. These pieces are about 3-1/2” long and 1” thick.
However, the origin of the glass is a controversial issue for the scientific community, with many evolving theories. Meteoric origins for the glass were long suspected, but recent research linked the glass to impact mechanics, such as vaporized quartz and meteoric metals, and to an impact crater. Some geologists associate the glass not with impact melt ejecta, but with radiative melting from meteoric large aerial bursts. If that were the case, the glass would be analogous to Trinitite, which is created from sand exposed to the thermal radiation of a fireball. To support the impact theory, the intense heat and pressure splashed melted sand up in the air and spread it over a large area of 4000 square kilometers. It created unusual shapes and small pieces of the original meteorite have been found imprisoned in the glass. A possible hint of iridium is an indicator for the extraterrestrial origin.
About 10,000 years ago, men discovered this glass. It was used as a substitute for flint. Artifacts can be found that have been shaped as sharp blades and graters. The Egyptians considered the glass to be precious and so it became part of religious objects like Tutankhamen’s amulet where there is a carved scarab made of this glass. Libyan/Egyptian desert glass is now extremely difficult to obtain. Removing material from the site is prohibited by the Egyptian government. This sample is 3-1/4” wide and weighs 121 grams.
The pieces are unusual when subjected to intense heat. Since impurities were burnt out of this material in a primordial ball of flame, it consists of almost a pure silicate. Glass can easily be melted at 2400 degrees. Moldavite starts to soften at 3400 degrees. Libyan desert glass starts to just surface soften at 4000 Degrees. It glows an intense solar white hot without any effects to the material.
![]()

One theory proposes that during the formation of the earth, a very large iron bearing asteroid struck the earth while it was in its molten stage. The asteroid merged to become the core at the center of the earth. Alternately, iron from the debris from space was drawn towards the center. In either case, most of the molten metals were pulled toward the center by gravity, which is why metals are so scarce in the earth’s crust. The core is composed mostly of iron and nickel plus other heavy elements However, iron meteorites have impacted long since then and have been found on or near the surface of the earth.
|
|
|
Here is a typical iron meteorite (left), perhaps similar in composition to the debris and/or asteroids that collided with the earth. This one weighs 4-1/2 pounds and came from a fall known as Campos Del Cielo in Northern Argentina, about 500 miles north-northwest of Buenos Aries. The time of fall was 4000 to 6000 years ago. It contains over 92% iron plus 6.68% Ni, 0.43% Co, 0.25% P, 87 ppm Ga, 407 ppm Ge and 3.6 ppm Ir. The crystal formations are shown at the right in a cross section and may have taken millions of years in a vacuum to form. The dark areas are carbon. Meteorites contain the element of Iridium, which is unique to these visitors from space.

![]()

Meteor Crater is located near interstate 40 in Northern Arizona near Winslow. It was created by the impact of an asteroid fragment traveling at 26,000 miles per hour and took place approximately 50,000 years ago. Today, Meteor Crater is nearly one mile across, 2.4 miles in circumference and more than 550 feet deep. It rocked the American Southwest with the energy of more than 20 million tons of TNT. I found these rocks just inside the rim. The rocks are about an inch across

![]()

This tektite is from Thailand and has a brownish translucence when held up to a bright light. The sample is 1” long. At that time it was thought that tektites were the result of an impact of a meteor on the moon. Some pieces of the resulting splash traveled to the earth. The Australasian strewn field is thought to be 800,000 years old. A part of one of the rock samples collected on Apollo 12, lunar sample 12013, has a composition which is remarkably similar to some tektites. Lunar Orbiter spacecraft images reveal fields of volcanic domes that may indicate deep-seated, high-silica eruptions on the Moon, possible sources of the tektites.
On the other hand, the terrestrial-impact theory states that a meteorite impact melts material from the Earth's surface and catapults it up to several hundred kilometers away from the impact site, which means that it must have travelled through space (thus explaining the dryness). The molten material cools and solidifies to glass. According to this theory, a meteorite impact causes their formation, but the precursor material of tektites is primarily of terrestrial origin, as determined from isotopic measurements.
![]()
Gold has been considered to be valuable for many thousands of years. Gold objects have been found in Bulgaria at least 6000 years ago. It has been prized for its rarity, beauty, easy workability and indestructibility. Besides the physical qualities of gold, the Egyptians valued it as symbolic of the gods. It also represents wealth, status and power.
Early use of gold was accomplished by finding nuggets and hammering them into shape without melting them. In the days of the pyramids, Egypt was known for being rich in gold. Rocks from the mines were broken up, ground and then panned the same as the way it was done in the gold rush days. Later, as technology improved, it was melted and often alloyed with other metals to give it greater strength and better malleability for use as jewelry, utensils or coins. We also know about the gold rushes in California, Australia and Africa.
Where does gold come from? It is formed literally from the stars. Stars like our own sun essentially convert hydrogen into helium. However, when the sun eventually collapses, it may only produce elements up to and as heavy as iron. Supernovae do emit some heavier elements up to nickel, lead, gold and even uranium. Current thinking is that this accounts for only 5% of the heavy elements. It is thought that infrequent collisions of neutron stars could account for the remainder. Neutron stars are so compact that they contain the mass of the sun in an area the size of a city. When the earth was formed, gold and the other heavy elements were included from the debris in space.

 Bacteria in the earth play an integral
role in creating deposits of gold. They can survive far below the ground where
there is no oxygen. Acids secreted by the bacteria break down the rock and
mineral ores. Molecules of gold are attracted to the outer skin of the
bacteria. Over the time of millions of years, the gold builds up to form a
glittering gold deposit. The process of continental drift by the earth's crust
and natural stratification may also contribute to forming veins of gold as well
as copper, silver and other elements that we find in the rock today. Gold is often
found embedded in quartz, shown at the left. This is typical of what could be
found in a gold mine.
Bacteria in the earth play an integral
role in creating deposits of gold. They can survive far below the ground where
there is no oxygen. Acids secreted by the bacteria break down the rock and
mineral ores. Molecules of gold are attracted to the outer skin of the
bacteria. Over the time of millions of years, the gold builds up to form a
glittering gold deposit. The process of continental drift by the earth's crust
and natural stratification may also contribute to forming veins of gold as well
as copper, silver and other elements that we find in the rock today. Gold is often
found embedded in quartz, shown at the left. This is typical of what could be
found in a gold mine.
Even today, gold can be found in creeks and hillsides as it was during the gold rush years and even before. Gold nuggets weighing a few grains to many grams, shown at the right, can be found by panning or with a sluice box. If you are lucky, you can still find large nuggets.
|
Chemical Symbol Au |
Atomic Number 79 |
A comparison with other elements
|
Element |
Atomic weight |
Density |
|
Iron |
56 |
7.9 |
![]()
|
|
|
Iron disulfide (FeS2) is a shiny brass-yellow mineral that is common. It is called pyrite or fool’s gold because it has a gold-like appearance and is often mistaken for gold. It can be found imbedded in rock as shown at the left or as a crystalline form shown at the right. It can also be found with impurities such as nickel, which is called Bravolite. Although pyrite contains much iron, it has not been as economical to mine as other iron ores.
The mention of fool’s gold reminds me of the movie The Treasure of the Sierra Madre (1948) with Walter Huston and Humphrey Bogart
![]()
 Radioactivity
occurs in unstable elements. Including variations, at least 76 have been
identified. These elements go through the process of decay. Depending on the
type of decay, they can emit alpha particles (helium nuclei), beta particles
(electrons) and gamma rays (shorter than x-rays) at very high velocities. (The
word radioactive is misleading and actually has nothing to do with radio waves)
Radioactivity
occurs in unstable elements. Including variations, at least 76 have been
identified. These elements go through the process of decay. Depending on the
type of decay, they can emit alpha particles (helium nuclei), beta particles
(electrons) and gamma rays (shorter than x-rays) at very high velocities. (The
word radioactive is misleading and actually has nothing to do with radio waves)
One of the most widely known radioactive substances found in nature is uranium.. We have all heard of the need to process uranium ore and concentrate it for use in power reactors or bombs. Uranium ore is typically very colorful as shown in the left photo. Uranium 238 (U238) continually decays into 15 other elements, including the intermediate elements of radium and radon gas, eventually ending up as an isotope of lead 206 (Pb206). This sample is still radioactive and will continue to be for a long, long time.
Radioactive materials are often referred to in terms of half-life. This is the time it takes for half of the material to decay. Although the half-life of U238 is 4.5 billion years, other steps in the process take only a fraction of a second. Radium 226 (Ra226) has one of the longer steps and has a half-life of about 1600 years.
Becquerel, a French physicist, found that uranium would affect a photographic plate. His associates, Pierre and Marie Curie, found that an ore called pitchblende had a higher activity than uranium oxide. In 1898, they were able to extract a minute quantity of a new element four million times as active as uranium oxide. They called it radium. It takes about 15 tons of ore to produce one gram of radium in the form of a salt.
![]()
Copper was first used by man over 10,000 years ago. A copper pendant discovered in what is now northern Iraq has been dated about 8700 B.C. For nearly five millennia copper was the only metal known to man, and thus had all the metal applications. Early copper artifacts, first decorative, then utilitarian, were undoubtedly hammered out from “native copper,” pure copper found in conjunction with copper-bearing ores in a few places around the world. By 5000 BC, the dawn of metallurgy had arrived, as evidence exists of the smelting of simple copper oxide ores such as malachite and azurite.
Not until about 4000 BC did gold appear on the scene as man’s second metal. By 3000 B.C., silver and lead were being used and the alloying of copper had begun, first with arsenic and then with tin. The blend of copper and tin forms bronze. For many centuries, bronze reigned supreme, being used for plows, tools of all kinds, weapons, armor, and decorative objects. Though copper came from the island of Cyprus-from whence its name-and numerous other sites in the Middle East, the origin of the tin in the bronze is still a mystery.
The Bronze Age suddenly ended at about 1200 BC, with the general collapse of the ancient world and the interruption of international trade routes. The supply of tin in particular dried up and the Iron Age was ushered in, not because iron was a superior material, but because it was widely available. The deliberate alloying of iron with carbon to form the first steels did not occur for centuries.
|
|
|
This ore from Arizona features the green color copper carbonate called Malachite. Rich copper minerals like this boost the copper content of this bonanza grade ore.

Michigan Native “Float” Copper
“Float” copper is found throughout the “Copper Country” of Michigan and nearby states. Glacial movements thousands of years ago altered the geologic deposits of the native copper by tearing and scouring the land. Copper, along with other rocks, gravel and sand were constantly tumbled and deposited over large areas in the Upper Midwestern United States. Exposure to the soils, water and air has oxidized the copper surface a bright green. The natural oxidation color provides a good contrast to the copper in this cross section.

At the left is a one pound ingot of .999 fine copper. Once copper has been concentrated, it can be turned into pure cathode copper in two different ways: Leaching & electrowinning or smelting and electrolytic refining. At the right is a copper tailing. Minerals are concentrated into a slurry that is about 15% copper. Waste slag is removed. Water is recycled. Tailings (left-over earth) containing copper oxide are routed to leaching tanks or are returned to the surrounding terrain.
![]()

This ribbon lava bomb is from the El Malpais National Monument near Grants, NM about 90 miles west of Albuquerque. It weighs about 8 ounces and is about 6” long. A volcanic bomb is a globule of molten rock ejected near the end of a volcanic eruption. As the neck of the volcano is being choked off by lava cooling around the opening, molten lava is formed into ribbon shapes as it is forced through the opening. This one appears to have been twisted when it was formed. Lava bombs can be thrown many kilometers from an erupting vent. They cool into solid fragments before they reach the ground. Ribbon bombs are often fragile and break easily when they fall back to earth.
![]()

Here are some tumbled and polished pieces of Obsidian. Obsidian is mineral-like, but not a true mineral because as a glass it is not crystalline. In addition, its composition is too complex to comprise a single mineral. It is sometimes classified as a mineraloid. Obsidian consists mainly of silicon dioxide (SiO2)), usually 70% or more. Crystalline rocks with obsidian's composition include granite and rhyolite. Because obsidian is metastable at the Earth's surface (over time the glass becomes fine-grained mineral crystals), no obsidian has been found that is older than the Cretaceous age. Obsidian has low water content when fresh, typically less than 1% water by weight but becomes progressively hydrated when exposed to groundwater, forming perlite.
Pure obsidian is usually dark in appearance, though the color varies depending on the presence of impurities. Iron and magnesium typically give the obsidian a dark green to brown to black color. Some stones have small, white, radially clustered crystals of cristobalite in the black glass that produce a blotchy or snowflake pattern (snowflake obsidian). Some obsidian may contain patterns of gas bubbles remaining from the lava flow, aligned along layers created as the molten rock was flowing before being cooled. These bubbles can produce interesting effects such as a golden sheen (sheen obsidian) or an iridescent, rainbow-like sheen (rainbow obsidian).
![]()

This volcanic ash sample is from Mount Saint Helens, WA, May 18, 1980. The fine-powdered ash sample was collected from Moses lake, WA. It is light gray in color. Small jagged pieces of rocks, minerals, and volcanic glass the size of sand and silt (less than 2 millimeters (1/12 inch) in diameter) erupted by a volcano are called volcanic ash. Very small ash particles can be less than 0.001 millimeters (1/25,000th of an inch) across. Volcanic ash is not the product of combustion, like the soft fluffy material created by burning wood, leaves, or paper. Volcanic ash is hard, does not dissolve in water, is extremely abrasive and mildly corrosive, and conducts electricity when wet.
Volcanic ash is formed during explosive volcanic eruptions. Explosive eruptions occur when gases dissolved in molten rock (magma) expand and escape violently into the air, and also when water is heated by magma and abruptly flashes into steam. The force of the escaping gas violently shatters solid rocks. Expanding gas also shreds magma and blasts it into the air, where it solidifies into fragments of volcanic rock and glass. Once in the air, wind can blow the the tiny ash particles tens to thousands of kilometers away from the volcano.
 At Mount St.
Helens, when the 2000 degree (F) lava emerges at the surface and comes in touch
with the ice, it melts in an instant, causing enormous steam explosions and
instantly transforms the lava into millions of tons of tiny light weight
particles of volcanic ash. For more than
nine hours, a vigorous plume of ash erupted, eventually reaching 12 to 16 miles
(20 to 27km) above sea level. The plume moved eastward at an average speed of
60 miles per hour with ash reaching Idaho by noon. Ashes from the eruption were
found collecting on top of cars and roofs next morning, as far as the city of
Edmonton in Alberta, Canada.
At Mount St.
Helens, when the 2000 degree (F) lava emerges at the surface and comes in touch
with the ice, it melts in an instant, causing enormous steam explosions and
instantly transforms the lava into millions of tons of tiny light weight
particles of volcanic ash. For more than
nine hours, a vigorous plume of ash erupted, eventually reaching 12 to 16 miles
(20 to 27km) above sea level. The plume moved eastward at an average speed of
60 miles per hour with ash reaching Idaho by noon. Ashes from the eruption were
found collecting on top of cars and roofs next morning, as far as the city of
Edmonton in Alberta, Canada.
Volcanic eruptions inject water vapour (H2O), carbon dioxide (CO2), sulfur dioxide (SO2), hydrochloric acid (HCl), hydrofluoric acid (HF) and ash into the atmosphere. HCl and HF will dissolve in water and fall as acid rain whereas most SO2 is slowly converted to sulphuric acid (H2SO4) aerosols. Ash particles may absorb these aerosol droplets onto their surfaces. When ash falls to the ground, the soluble components (leachates) can by washed away by water, potentially resulting in changes to local water chemistry and hence quality. Chemical changes in the underlying soil can occur as a result of leaching of the aerosols coating individual grains and longer term from unstable glass particles.
![]()

There was an unusual formation in a cliff near where I used to work in upstate New York. I took some samples to save. In the picture, you can see that the stones and sand are held together with an orange-whitish material. The book describes this as a conglomerate of glacial till composed of rounded pebbles 1\4” across or larger, cemented together in a matrix of finer material. The cement may be iron oxide, silica or calcium carbonate. The coarse materials are deposited close to shore at the mouths of swift rivers in alluvial fans or in deltas. The material was laid down in valleys by rivers that drained away from the glaciers, and in glacial lakes. The location of the conglomerates is cited as New York State. This glacial conglomerate was formed in the last ice age about 11,000 years ago, along with the Finger Lakes, Great Lakes, Cape Cod, Nantucket and Martha’s Vineyard.
The rounded stones, of course, indicated that they had been tumbled by the glacial river over a long period of time. As the river subsided, the water evaporated from the edges, concentrating the binder that was dissolved in the water so it would become a solid and hold the gravel together all of those years.

![]()

There were many unusually shaped rocks in my creek. One day I found this interesting stone measuring about 8” by 10” and 3” thick. In the center was a rounded red stone about 6" across embedded in the gray slate-like stone. The front face of the red stone is exposed, just as I found it.
|
|
|
The back was all gray indicating that the red stone was very thin. I had coated this with shellac to make the color more visible To this day I don't know how it was formed. Of course, a rare find like this could not be left there.
|
|
|
After carrying it for about 1000 feet down the length of the creek bed, I was very curious to find out how much it weighed. It became very heavy and my arms were sore the next day. It was 17 lbs. on the bathroom scale, a reminder about not being overweight.
A geologist at Cortland suggested the red stone may have been formed by an air pocket that later filled with red sediment. That's probably more believable than thinking someone or something threw it there in the prehistoric mud. Of course, it may have just fallen in the ancient sediment and then covered by more sediment later on.
![]()

Fossils could be found lying on the ground anywhere in my woods but the creek was the best place to find them. This 12” long specimen was near the lower path. I wondered how long it had been there and where it had come from, perhaps from further up the hill. Incredibly, the sides looked like they had almost been cut with a diamond saw. It was also too good to just leave there. I had coated this with shellac to bring out the texture. I also found two slabs that were about a square foot in size and 2-3” thick. These were also very heavy.

The presence of fossils indicated that this area was once under water and perhaps from an ancient sea bed from the Mississippian to Permian age and as old as 345 million years. Most of the fossils were brachiopods and crinoid stems. The little serrated disc fossils had me puzzled for a while until I learned they were part of a crinoid, and invertebrate animal. The stem of this plant was made of these discs which were stacked one above the other. When the plant dies, the discs all separate.
![]()
 Here is a
specimen from a leg bone fossil shown at the left. It is from the Morison
Formation in the Colorado Plateau. It was part of a living animal during the
Jurassic period around 144 to 208 million years ago. This bone has calcite
crystal cells with a reddish brown cell wall matrix. The end has been cut to
show the cross section. It weighs 19 ounces and is 3.25” X 2.75” X 3”
Here is a
specimen from a leg bone fossil shown at the left. It is from the Morison
Formation in the Colorado Plateau. It was part of a living animal during the
Jurassic period around 144 to 208 million years ago. This bone has calcite
crystal cells with a reddish brown cell wall matrix. The end has been cut to
show the cross section. It weighs 19 ounces and is 3.25” X 2.75” X 3”
This fossil is radioactive but not all fossils might be radioactive. One clue is that it can be very colorful and here it has orange and red colors. The amount of radioactivity depends on what minerals were in its surroundings when it was fossilized. Although the radiation is very minor in this bone sample, it is measurable with a Geiger counter placed against the cut face (about 0.13mr/hour). The same process can apply to teeth as well.
![]()
 This Spinosaurus tooth was found in
Morocco at the fringe of the Sahara Desert and shows a very low level of
radioactivity. This piece also confirms what I had heard about dinosaur fossils
being radioactive.
This Spinosaurus tooth was found in
Morocco at the fringe of the Sahara Desert and shows a very low level of
radioactivity. This piece also confirms what I had heard about dinosaur fossils
being radioactive.
Spinosaurus (meaning "spine lizard") is a genus of theropod dinosaur which lived in what is now North Africa, from the lower Albian to lower Cenomanial stages of the Cretaceous period, about 112 to 97 million years ago. This genus was first known from Egyptian remains discovered in the 1910s and described by German paleontologist Ernst Stromer. The original remains were destroyed in World War II, but additional material has come to light in recent years. It is unclear whether one or two species are represented in the fossils reported in the scientific literature. The best known species is from Egypt, although a potential second species has been recovered from Morocco.
Spinosaurus may be the largest of all known carnivorous dinosaurs, even larger than Tyrannosaurus rex and Giganotosaurus. Estimates published in 2005 and 2007 suggest that it was 12.6 to 18 metres (41 to 59 ft) in length and 7 to 20.9 tons (7.7 to 23.0 short tons) in weight. The skull of Spinosaurus was long and narrow like that of a modern crocodilian. Spinosaurus is thought to have eaten fish; evidence suggests that it lived both on land and in water like a modern crocodilian. The distinctive spines of Spinosaurus, which were long extensions of the vertebrae, grew to at least 1.65 metres (5.4 ft) long and were likely to have had skin connecting them, forming a sail-like structure, although some authors have suggested that the spines were covered in fat and formed a hump. Multiple functions have been put forward for this structure, including thermoregulation and display. The “sail” is similar to the much earlier animal called the dimetredon.
Spinosaurus appeared prominently in the 2001 film Jurassic Park III. The film's consulting paleontologist John R. Horner was quoted as saying: "If we base the ferocious factor on the length of the animal, there was nothing that ever lived on this planet that could match this creature [Spinosaurus]. Also my hypothesis is that T-rex was actually a scavenger rather than a killer. Spinosaurus was really the predatory animal." In the film, Spinosaurus was portrayed as larger and more powerful than Tyrannosaurus: in a scene depicting a battle between the two resurrected predators, Spinosaurus emerges victorious by snapping the tyrannosaur's neck. In reality, such a battle could never have taken place since Spinosaurus and Tyrannosaurus lived thousands of kilometres apart (what is now North America vs. what is now Africa) and millions of years apart. This battle, if it were to actually have taken place, is the subject of a controversy for which would be the victor. In the film, it can be seen that the T-Rex first had the Spinosaurus by the neck. Knowing the T-Rex had much more powerful jaws, it should have been able to crush the neck of the Spinosaurus and end the battle right away. However, that would not have been in keeping with the entertainment factor. Spinosaurus was also featured in merchandise related to the Jurassic Park films, including action figures and video games.

![]()

This Elasmosaurus tooth fossil was found in the Khouribgha phosphate mines in Morocco. In the matrix of rock on this item are some partial Enchodus teeth and some other bone fragments which is an interesting feature. These were aquatic dinosaurs that lived in the late Cretaceous period, around 70 million years ago. This dinosaur had an extremely long neck, a large body, and 4 flippers. It had a relatively small head and many sharp teeth.

Versions of “Nessie” the Loch Ness Monster have been modeled after this dinosaur.
![]()
While vacationing in Chatham on Cape Cod, I visited a rock shop in town. I learned from the owner that he would be interested in swapping some fossils I found in my woods and along the Chenango River for some of his. One that I really liked was an Ammonite that he said came from Morocco in Africa.
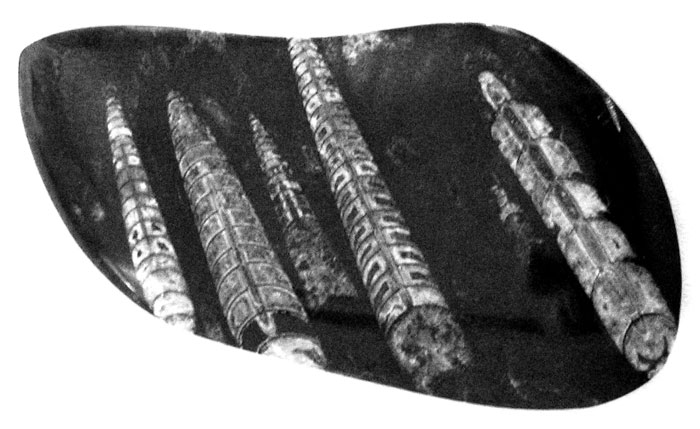
I later learned that these are straight-shelled cephalopods, known as Bacrites (Baculites) and were a predecessor to the spiral ammonites. They first appeared 415 million years ago. Baculites lived throughout the world during the Late Cretaceous Period. They ranged in size from seven centimeters up to two meters. Distant relatives are the cuttlefish, squid and nautilus.

Ammonites are also cephalopods and first appeared about 240 million years ago. Ammonites were predatory, squidlike creatures that lived inside coil-shaped shells. Like other cephalopods, ammonites had sharp, beaklike jaws inside a ring of tentacles that extended from their shells to snare prey such as small fish and crustaceans. Some ammonites grew more than three feet (one meter) across.
Ammonites constantly built new shell as they grew, but only lived in the outer chamber. They scooted through the warm, shallow seas by squirting jets of water from their bodies. A thin, tubelike structure called a siphuncle reached into the interior chambers to pump and siphon air that helped move them through the water. Ammonites were prolific breeders, lived in schools, and are among the most abundant fossils found today. They went extinct with the dinosaurs 65 million years ago. Scientists use the various shapes and sizes of ammonite shells that appeared and disappeared through the ages to date other fossils.

Some ammonite fossils bear intricate patterned details on their outer surface called Sutures. These are located beneath the external shell wall, and are often visible if the fossil has been subject to weathering or artificial polishing. These patterns mark where the walls of the chambers, Septum, meet the outer wall of the ammonite shell. This method of construction is thought to have provided strength to the shell when diving to deeper depths. Suture patterns are very useful for distinguishing different species of ammonite.
Ammonites were the predators of their time, feeding on most living marine creatures including mollusks, fish and even other cephalopods. By analogy to modern cephalopods, their method of attack probably comprised of silently stalking their prey, then rapidly extending their tentacles to grasp the target. Once caught the prey would be devoured by the ammonite's powerful jaws, located at the base of the tentacles, between the eyes.
Much of the ammonite's life was spent in shallow waters. The evidence to support this includes their diet, which could be found in the greatest volumes in the warm shallows. It is unlikely that their shells could withstand the high pressures present in deep water (over 100 meters). Other theories based around their social behavior suggest their shells were decorated by an array of patterns or color that might have played a large part in their lives. They typically lived for two years, although some species survived beyond this and grew very large. Evidence of their short lives is estimated by looking at their living relatives - the nautilus. These creatures exist within modern day seas and possess many characteristics similar to ammonites (see picture below).
![]()

A favorite food of the Ammonites was the trilobite. There were many hundreds of trilobite variations over millions of years from the Cambrian to the Permian ages. They ranged in length from a fraction of an inch to about nine inches and were probably bottom feeders and predators. The body had three main sections that led to the “tri” portion of the name. Most had two eyes and two antennae.
![]()

I bought this Nautilus shell about 1980 at Disneyworld. The name comes from the Greek nautilus meaning sailor. The chambered nautilus is believed to be the closest living relative to the ammonites. During prehistoric times there were 10,000 different species of nautilus, but only two different species survive today. They haven’t changed much in 500 million years so are considered “living fossils”. The nautilus lives in the Indian Ocean and the tropical western Pacific Ocean. During the day they are at depths of 1800 feet. At night they rise and travel to coral reefs to feed; they eat crustaceans. When swimming the nautilus can reach speeds over two knots.

Their shells are made of mantle tissue and closely resemble the ammonites shell with some differences. Ammonites were able to go inside their shells and close a plate to protect it from other predators. The Nautilus has a leathery head shield called a hood to protect them when they go inside their shells. The Nautilus also, has the ability to camouflage itself. If you look down from the top, the shells closely spaced coloration blends in with the darkness below. If you look from underneath, the shell the coloration is widely spaced white blending in with the light from above. The Nautilus has poor vision. Even though the eye structure is highly developed; they don’t have a solid lens. Instead, they have a pinhole lens that water can pass through to equalize pressure. Ammonites have 26 chambers. Nautilus have 30 chambers and have anywhere from 38 to 90 tentacles.
Nautilus shells have individual chambers, each growing in size as the creature grows. These chambers are secreted by the creature at a rate of one every four weeks, equal to 13 each year. Using this as a guide an ammonite shell containing 26 chambers could be assumed to have housed the creature for two years. Like the nautilus, ammonites retained their original shell throughout their life. In comparison to modern day nautili live in cold, deep water whereas ammonites preferred warm shallow waters.
![]()

These pieces of slate I picked up in Llanberis located in the Snowdonia National Park, Northern Wales. The slate distinctly covers the mountains where it has been quarried creating steep drops or slippery slopes and gleaming in the rain. It had been raining the day I picked them up.

Llanberis is now a popular tourist area and includes a railway to the top of the mountain. The Snowdonia National Park takes its name from Snowdon which, at 1085m (3,560 feet), is the highest peak in Wales and England. In Welsh, Snowdon used to be called Yr Wyddfa Fawr (the Great Tomb or the Great Throne) or Carnedd y Cawr (the Cairn of the Giant). Nowadays it is simply called Yr Wyddfa, but the various names bear testament to a land steeped in legends, history and tradition. This is the ancient Kingdom of Gwynedd, the heart of Wales and the stronghold of 'Cymraeg', the Welsh Language. The Welsh name for the National Park is Eryri (The Highland).
![]()
Flint

Flint was used for the manufacture of flint tools during the Stone Age. It splits into thin, sharp splinters called flakes or blades (depending on the shape) when struck by another hard object (such as a hammerstone made of another material). This process is referred to as knapping. I found these pieces of flint in a parking lot at Windsor Castle in England. Flint was used in the construction of many churches, houses, and other buildings. It has been used to the present day as a material for building stone walls, using lime mortar, and often combined with other available stone. It was most common in parts of southern England, where no good building stone was available locally, and brick-making was not widespread until the later Middle Ages.

I noticed that pieces of flint were knapped and then cemented in place in rows along the tops of the walls around the castle perhaps to discourage invaders. Many different decorative effects have also been achieved by using different types of knapping or arrangement and combinations with stone (flushwork), especially in the 15th and early 16th centuries.
![]()

I found these limestone pieces on the grounds of Blenheim Palace in Oxfordshire, UK. Due to its formation, the limestone in the Oxfordshire area is referred to as inferior oolithic limestone. The inferior oolite has been used as a building stone in many picturesque villages and towns of the Cotswolds, as well as the Oxford colleges and great buildings such as Blenheim Palace. The limestone has a yellowish-white color on extraction which weathers to a lovely honey color, evident throughout Oxford.

Blenheim Plalace
The limestone formation in particular is important; it was formed by the chemical precipitates of calcium carbonate from organisms known as ooliths. Ooliths still grow today in water less than 5meters deep in areas with strong currents and this helps us understand what the conditions were like over Oxford during the middle Jurassic period.
![]()

 This is the pyramid at Chichen Itza,
Mexico. Chichen was founded by the Maya civilization about 400 AD and it is
located in the north of the Yucatan Peninsula.. This is not far from Merida and
Progresso where a large asteroid impact may have contributed to the demise of
the dinosaurs. Dominating the center of Chichén is the Temple of Kukulkan (the
Maya name for Quetzalcoatl, often referred to as "El Castillo" (the
castle). This one side of the temple has been restored. Here I am sitting on
the shallow limestone steps. I decided not to go to the top. They are very shallow
and are deliberately made that way.
This is the pyramid at Chichen Itza,
Mexico. Chichen was founded by the Maya civilization about 400 AD and it is
located in the north of the Yucatan Peninsula.. This is not far from Merida and
Progresso where a large asteroid impact may have contributed to the demise of
the dinosaurs. Dominating the center of Chichén is the Temple of Kukulkan (the
Maya name for Quetzalcoatl, often referred to as "El Castillo" (the
castle). This one side of the temple has been restored. Here I am sitting on
the shallow limestone steps. I decided not to go to the top. They are very shallow
and are deliberately made that way.
During those earlier days, it was governed by priests. Chichen Itza means “At the mouth of the well of Itza”. Itza stands for ‘the Itza tribe’. The main belief was that people were thrown from the top as a sacrifice to make their god happy and the ones who could survive were the ones who were believed to be seers.

I found these rocks at Chichen. The top one in the picture is from the field area around the pyramid. The bottom two were found near the base of the pyramid. They are composed of limestone. Limestone is a sedimentary rock composed largely of the minerals calcite and/or aragonite, which are different crystal forms of calcium carbonate (CaCO3). Limestone is soft when quarried but hardens after exposure to air. The Mayans used chisels made of flint to cut the limestone. Flint inclusions were often found in the limestone and used as part of the design. The great pyramids in Egypt were also made of limestone.
![]()
Here are some
sand samples we collected from various beaches.
The sand can vary a lot and depends on which beach and sometimes which area on
the beach that the samples are taken.

Perhaps this suggests a painting of mountains in the distance with mist rising from the valleys. Alternately, this could be ocean waves and mist early in the morning. For me it was a typical area on the beach in Chatham on Cape Cod. My mother used to call it iodine sand but I never knew the reason why. It has sort of a purplish tint. These darker grains were washed up on the beach by the waves as the tide went out leaving an imprint of the last spent wave. The lighter grains form the outline of the hills and the misty areas. Not all beaches have the darker grains and even those only seem to have certain areas where they show the most. This picture represents about two feet of beach width

Of course, I took some home with me to remember. Recently, I gained the opportunity to photograph the composition of the sand and try to make a connection to how it became separated from the rest of the grains that form the majority of the beach sand. It became obvious that the darker grains seem to be smaller and perhaps lighter than the rest. Medium-grain granite is known to have mostly quartz (SiO2) with other minerals like feldspar and biotite mica. Fine grained granite has feldspar that my redden it. The composition of the dark grains is not certain. However, biotite mica is brown or black containing magnesium and iron. However, none of the grains in this sand are attracted to a magnet.

The majority of sand on the Chatham beach looks like this. It appears to contain lots of quartz and some white feldspar plus a few other minerals. According to a book about the geology of the cape, the sand was formed by glaciers that ground up granite from the area of Maine and brought it south. The moraine was deposited over the years. At one period of time about 11,000 years ago, the forward edge of the glacier melted as fast as it advanced piling up all of the material than formed Cape Cod, Nantucket and Martha’s Vineyard.
![]()
Acadia Beach Sand

I found this sand on a beach in Acadia National park along the coast of Maine. Individual grains of translucent, gray, glassy-looking quartz and pink or gray feldspar are easily seen. The black mineral is hornblende. Shell fragments and other grains can also be seen.

This is the view from Cadillac Mountain in Acadia.
Steep slopes rise above the rocky shore, including Cadillac Mountain, which at 1,530 feet is the highest point on the U.S. Atlantic coast. One of the most spectacular geologic features on the island is the shatter zone that surrounds the Cadillac Mountain granite on nearly all sides and ranges in width from a thousand feet to more than a mile. The exposed ledges are made of a pink to greenish-gray, coarse-grained hornblende granite that contains minor biotite. The rock is uniform in appearance.
![]()
Central Florida Sand

This sample I dug up from my back yard in Central Florida. After digging down about 2 feet, the sand is almost all white

![]()
New Smyrna Beach, FL Sand

![]()
Clearwater, FL Beach Sand

This sample is from a beach in Clearwater, Florida.
![]()
Sarasota, FL Beach Sand

Half Moon Key, Sarasota, FL
Although located only a few miles away, this beach fine grained sand is much different from Clearwater.
![]()
Cancun, MX Beach Sand

This sample is from a beach in Cancun, MX. Due to erosion from storms, the beaches are gradually washed away. They are periodically restored by pumping sand in from offshore sand bars to compensate for the loss. The sand has an unusual feel of softness and is cool to walk on because it is composed of microscopic fossils that do not absorb heat. All of the grains are rounded and many of them are very small.

Here is a view of some of the hotel beaches in Cancun that have been restored.
![]()
Hawaii Beach Sand

This is beach sand from Hawaii. According to one source, very little of this sand is from volcanic rock. It may have come from the Kona Coast of the Big Island. Hawaii has no natural source of quartz and so that is not present in the sample. Much of the remainder is composed of the carbonate shells and skeletons of marine organisms, such as corals, algae, mollusks, foraminifera, echinoderms, and bryozoans that contribute to the yellow, orange and white grains. Other beaches can have black sand from volcanic rock and white sand from shells of marine organisms.
![]()
Costa Rica Beach Sand

This beach sand is from Costa Rica on the Pacific side. The sand is very dark. Some of the granules are attracted to a magnet indicating that iron is present. An active volcano is about 20 miles away and a major eruption occurred in 1968.

![]()
Bermuda Beach Sand

A beach in Bermuda
![]()
Grand Cayman Beach Sand

Seven Mile Beach on Grand Cayman
![]()

One of the more eye-catching finds on the beach is broken glass that has been rounded and smoothed by the action of the waves. Although this glass is not as common on Cape Cod beaches, I have found it more plentiful along New Jersey beaches such as Asbury Park. The glass varies in size but is commonly found about 1/2” to 1” The glass appearance is frosted from abrasion. The most common is clear glass then green, brown blue and red. In a way, it is as much fun finding these as it is finding valuable treasure in the sand.

These are small stones I found along a Chatham beach and grouped according to color. They are all about 1/4” to 3/8” in size. Eventually I expect they will be ground down into smaller pieces by the waves.
![]()

Along with the small stones, shells are a part of the beach sand and are also rounded by the waves. These particular shells are from quohaugs. That is a common name in New England but may also be called cherrystone clams elsewhere. The purple coloring is characteristic of a portion of the inside of the shell. These particular pieces measure about 1” in size but the whole shell can be up to 4” or so across and is very thick. In time, they would probably be ground down as well. I wonder if there is a connection with grain-sized pieces and the purple “iodine” sand.”
![]()

This one is probably quartz. I found it while walking along the Nauset Light beach at Cape Cod. It was still partly buried in the sand and being washed by the waves. Large rocks were relatively scarce and white ones were even scarcer. This large one is about 8” across and 5” high and weighs about 15 pounds. I was holding it on my shoulder to carry it back along the beach when a woman asked me if I wanted this stone. I said yes and continued on. I wonder what she would have said if I had asked $5 for it and would include carrying it back to her car. A year later I found another slightly smaller one to go with it. I have had these rocks for more than 30 years now.

Nauset Light Beach consists of a broad, sandy beach that is backed by steep cliffs of glacial sand and a few rocks. Each year, the cliffs are eroded away. In 1996, the light house was moved 300 feet further inland. The sand and rocks that can be seen today were transported and deposited by the last glacier about 10,000 years ago and have never seen the light of day until exposed again by erosion.
![]()
|
|
About This Site |
|
|
|
More text and pictures about my rock collection will be added as my search continues. Any comments, corrections, or additions are welcome. |
|
|
|
Created
by Roger Russell |
Links
http://www.microscope-microscope.org/applications/sand/sand-links.htm









